Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124

How to cite:
Wong M. Investigating the viral heat protectant test. Lab Muffin Beauty Science. September 9, 2025. Accessed September 9, 2025.
https://labmuffin.com/investigating-the-viral-heat-protectant-test/
You might’ve seen this viral heat protectant test. You spray a heat protectant on a thermal paper receipt, then use a flat iron hair straightener on it. Since receipt paper turns black with heat, the receipts that stay white have the more effective heat protectants:

… Or so the logic goes, anyway. But does this test actually work, and if not, what’s really going on?
This question turned out to be much more complicated than I thought.
The video version of my investigation is here. It didn’t translate well to text, so this article is a simplified version of the process, but has more experimental details – watch the video to see the true messy scientific process in action!


Most of the time, it’s easy to dismiss testing beauty products on objects that aren’t skin or hair as invalid (I go through a lot of examples here). Your skin isn’t an apple, your lips aren’t paper – the list is endless.
But this test has a bit more surface validity. Hair gets damaged by excessive heat, receipts have an ink on the front that goes darker with heat, so blacker receipts should mean more heat and hence more damage.
In science, we often test on models – something that has similar enough characteristics to the real thing to give us useful information. We test medications on mice, and skincare on little chunks of skin (e.g. EpiSkin, a reconstituted human skin model developed by L’Oreal – it’s replaced a lot of animal testing).
But if the model is too different, it can be useless. So the question is: are the receipts changing colour when hair gets too much heat?
I started by watching the most popular series of videos of this test from Lucy Seitz, a cosmetologist. She’s quite systematic in her tests, stating the temperature of the straightener (340 °F) and using a control.
It looks like the receipts in her videos are still wet. When a commenter pointed this out, she modified her tests to add a drying step. But it looks like something is still dripping off a few of the receipts:


This could explain what’s happening: some receipts have more product on them, which could soak up the heat before it reaches the receipt. If it’s water, this could have a significant cooling effect – water is one of the best common substances for soaking up heat, due to its hydrogen bonding.
My first experiment was to try to replicate her tests, so I could make more observations for myself. I found 11 products that had heat protection claims, with a range of formulas so I could hopefully see some sort of pattern:
7 pump sprays:
1 propellant spray:
3 cream products:
The only product Lucy used that I had on hand was the Marc Anthony spray. After spraying it on a receipt, I used my straightener on it (set to 170 °C, close to what she used)… and it crackled and smoked.
Change of plan – I decided to apply products to all the receipts, let them dry for a bit (however long it took to apply all the products – turned out to be around 15 minutes), and then test them. I didn’t control the amount of product I applied, to better replicate Lucy’s tests as well as normal application (plus it was faster!).
There’s a huge difference between 0 minutes and 15 minutes with the same product – so drying clearly can make a big difference.


The lightest receipts are the ones with creams. This makes sense because creams would apply more thickly than sprays, similar to sunscreen lotions vs sprays. More product would mean the receipt is more insulated from heat, plus they would dry slower, so more water might be present.


The darkest two receipts are the Goldwell and IGK sprays. This also makes sense since these are the two products that only have water low on the ingredient list, so they wouldn’t be buffered as much from heat.
It’s difficult to see how light the rest of the receipts are, since the product application wasn’t controlled. There was no obvious pattern with the products or ingredients, so it could just come down to how much product was applied, and slight differences in how long they had to dry.


To test whether the different results are just due to different amounts of cooling from water, I dunked and sprayed a bunch of receipts with water, then applied a straightener set to 170 C immediately, after 2 minutes, 5 minutes and 10 minutes.
Dunking: The receipts have a lot of white at all tested times. Interestingly, the receipt seemed to be less white when tested immediately. This might be because the water didn’t have time to soak into the middle of the paper yet, so the cooling effect wasn’t as strong.


Spraying: The receipt at 0 minutes has white bits, but it’s grey by 2 minutes and black by 5 minutes.


Water clearly makes a difference! Generally, the more time the receipt had to dry, the darker it was.
A small amount of water can do a lot of cooling, and paper is relatively hydrophilic, so it could hold onto water pretty well. But the effect isn’t that big with the smaller amount of water from a spray. However, heat protectants usually contain humectants which would slow water from evaporating, so it’s possible there could be enough water after 5 minutes for a cooling effect.
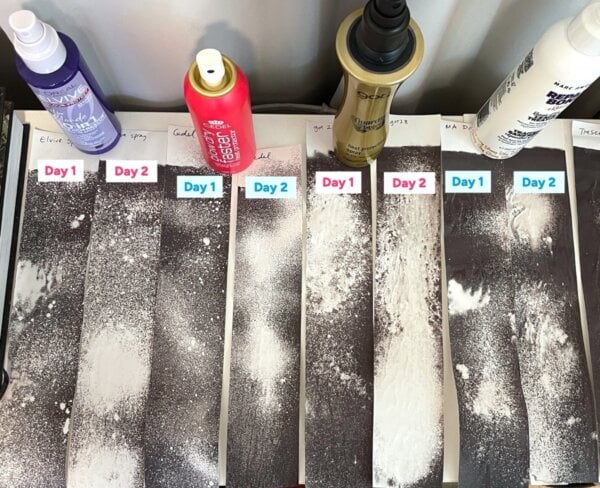
I also applied product to a second set of receipts on Day 1, so I could also test them 24 hours later, when they were thoroughly dry. Since I assumed water was cooling the receipts, I expected these to all be much darker… but they very much weren’t!





At this point, I was considering a lot of time-consuming experiments (Testing different ratios of glycerin and water? Formulating some simple heat protectant bases?), so I started researching thermal paper structure. This turned out to be quite difficult, since it seems to be a trade secret.
By chance, I ended up on the German Wikipedia article for thermal paper, which referenced a German paper. While reading the Google Translated version, I came across the melting point of the substances in thermal paper: 40 to 80 °C.
This was a huge surprise to me. At the start, I googled “thermal paper temperature”, which returned a single numerical result in the first few pages: 150-185 °C, from a packaging company. This is similar to a hair straightener, and I didn’t check too thoroughly since I assumed this was in the right ballpark. 40-80 °C is far, far lower.
It turns out, searching “what temperature does thermal paper change colour” gets completely different results with lots of different temperatures, because Google is freaking broken.
The most legitimate-looking result (from a thermal paper company):
“Thermal paper begins to develop colour at between 60 to 100 °C (140-212 °F) and reaches applicable density between 70 and 120 °C (158-248 °F)”
This threw a huge spanner into the works. If receipts change colour at the lower end of this range – say, 50 °C – then this test would be completely useless. Hair doesn’t get damaged until around 100 °C, so the receipt would change colour much earlier.
To quickly check this, I poured boiling water onto a piece of the receipt paper I’d been using – it changed colour, so the temperature is definitely under 100 °C. But by how much?
To work out the temperature at which receipts change colour, I cut up a bunch of different receipts from stores and stuck them together, then dipped them into a beaker of boiling water that had been allowed to cool to different temperatures (water is great at keeping a stable temperature – I also tried heating them on a hotplate and checking the temperature with an IR thermometer, but the temperature was too uneven and the receipt didn’t have enough contact with the hotplate to give reliable results.)
This test also turned out to be more complicated than expected (a recurring theme). Paper bits fell off the tape when they got wet, and then when I tried to encase the bits in sticky tape, the adhesive seemed to keep the receipts white. Eventually, I stuck the receipts face down onto a thin piece of plastic wrap, giving us a clear result: most receipts turned black around 95 °C. So this part of the test, at least, matches the temperature needed for hair damage.


At this point, some of the observations could be explained with the original hypothesis: Some products “work” in this test because residual water was evaporating and cooling down the receipts:
Blow drying the receipts probably can’t get rid of all the water (some of Lucy’s receipts seem to be producing steam).
This already shows the test isn’t that good. Water is a terrible heat protectant because it soaks into the hair, then can explosively evaporate when heated with a hot tool, much like popcorn (“bubble hair”):


And reducing heat isn’t the point of a heat protectant – it’s meant to help the heat spread out more evenly, and reduce damage from tools. A metre of air is a great heat protectant, but your hair isn’t going to change shape without heat (the whole point of using a hot tool in the first place).
But there were also a lot of observations that residual water could’t explain:
The receipts turning grey right after spraying was especially surprising to me, because I hadn’t noticed this happening in other people’s videos… so I went back and looked more closely at Lucy’s tests.
There were some grey receipts. It’s subtle, and it’s sometimes hard to tell if it’s just a shadow, or just wet, but some were definitely grey. And the edges of the grey sections lined up with where the receipts stayed white after heating:


They were nowhere near as grey as my receipts, but after playing around with some receipts and the IGK spray I worked it out: the spray was erasing the ink after it formed. Lucy used a lot more spray than me, so her receipts mostly went back to white!
The sprays that turned the receipts grey had high amounts of alcohol. Alcohol is a good solvent that can dissolve oilier substances than water, which led to my new hypothesis: Heat protectants that “work” are dissolving the ink layer on the receipt.
Thermal receipt paper has a special ink layer on one side that’s in multiple invisible parts, frozen in a solvent (sort of like wax). When heat melts the solvent, the parts come together to form new black ink, sort of like how separate ice cream flavours can melt and mix to create a new colour. The ink is fragile – it only forms under specific conditions (you can see the black disappearing if you reheat the receipt).
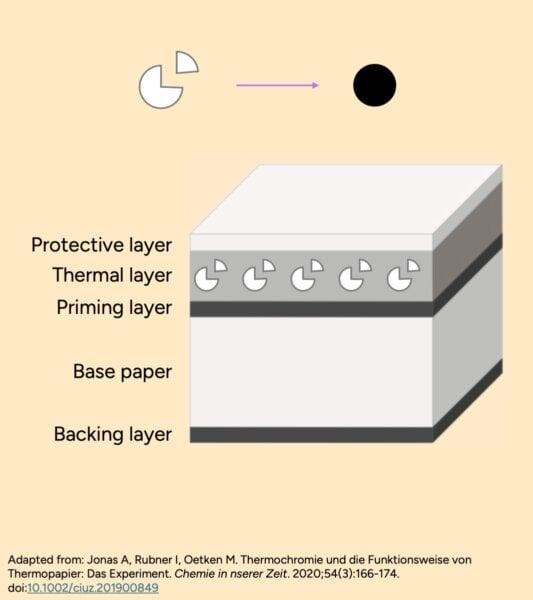

Alcohol dissolves the ink layer, so the components can come together and form some grey ink. But more alcohol dissolves the layer too much, messing up where the ink components are, so they can’t come together and form the dark ink anymore – heat protectants that keep receipts white could be doing this too.
To check my hypothesis, I tested a bunch of substances that definitely weren’t forming a protective layer.
Alcohol evaporates faster than water. Drops of alcohol (methylated spirits), alcohol with water, alcohol with water and glycerin all kept the receipt light (all these receipts were heated after about 30 minutes of drying):
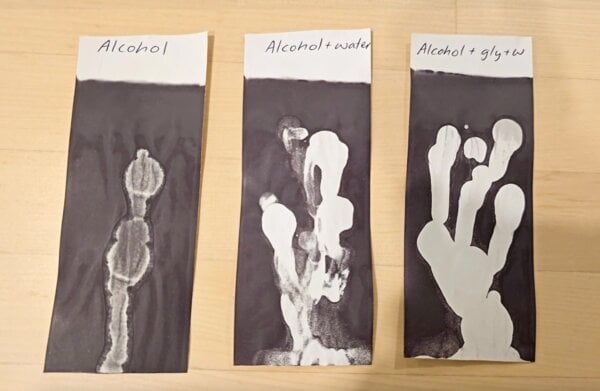

Diluted alcohol made the receipt whiter than the alcohol alone, which makes sense if it’s dissolving the ink layer. Alcohol evaporates really quickly, but water and glycerin can hydrogen bond with the alcohol so it stays on the receipt for longer. This gives it more time to mess the receipt up (hydrogen bonds also why sanitising surfaces with a 70% alcohol in water solution works better than pure alcohol).
This also explains Weird Observation 2, where spray products looked lighter on Day 2: the products kept messing up the ink after the first 15 minutes.
I also tested water alone, and water with glycerin. They didn’t disturb the ink much, which makes sense since receipt inks would be too oily for them to dissolve.


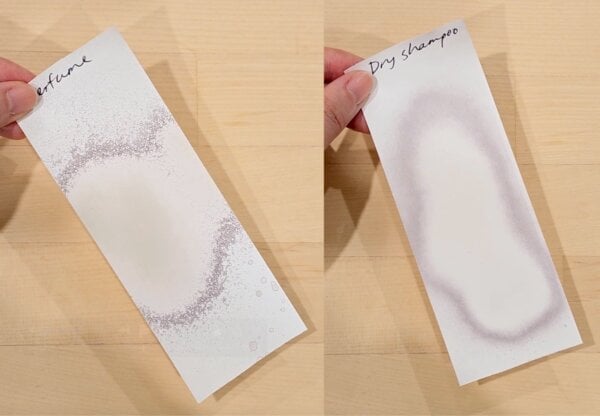
Other products with a lot of alcohol should also mess up the receipt. Perfume made the receipt immediately grey, then kept it white with heating.
Dry shampoo made the receipt immediately grey, but didn’t keep the receipt as white when it was heated. These don’t contain much humectants, so the alcohol wouldn’t be staying on the receipt for long enough to do much dissolving.



Alcohol explains some of the receipt results, but a lot of heat protectants that “worked” didn’t contain alcohol. They do contain surfactants though, which are also good at dissolving things – surfactants are the ingredients in cleansers that dissolve oil off your skin.
Drops of micellar water, and water with detergent kept the receipts white when tested around 30 minutes after application:


There are also surfactants (emulsifiers) in other beauty products like moisturisers, to keep the oil and water parts mixed together. I tested a cream (CeraVe Moisturising Cream) and a cream spray (Laneige Cream Skin) – both kept the receipt paper white:
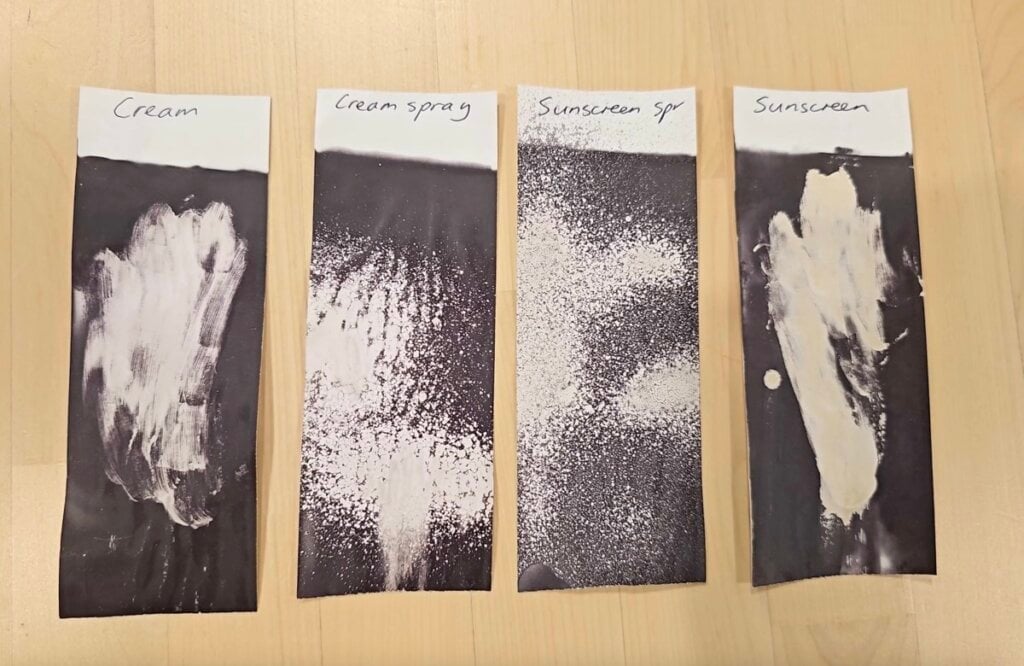

Sunscreens often contain both alcohol and surfactants – both a sunscreen (La Roche-Posay Anthelios UVMune 400 Fluid) and a sunscreen spray (Naked Sundays) kept the receipts white.
These interactions with alcohol and surfactants explain why some sprays go lighter than a lot of creams, but some are really dark. Sprays that have alcohol tend to have a lot, but some don’t have any. All creams have emulsifiers, and rubbing them onto the receipt helps mess up the ink layer more.
This also explains what’s happening with sticky tape – the adhesive sometimes contains surfactants or solvents.
I also tested the top silicone layer of a two-phase eye makeup remover that was mostly cyclopentasiloxane. This didn’t have much impact on the receipt, probably because it’s a bit too oily to dissolve the ink layer, plus it evaporates too quickly. This would explain why some silicone-based products “failed” the receipt tests.


I also noticed that some of Lucy’s products that worked really well had HFC-152a as the propellant, and this could potentially do something to the receipts. I tried compressed air, which is pretty much just the propellant – this had no effect.


If the ink layer is indeed being dissolved by these products, I should also be able to erase the dark ink by messing up the layer with the same substances. I tested alcohol, perfume, sunscreen, micellar water and moisturiser – these largely worked!
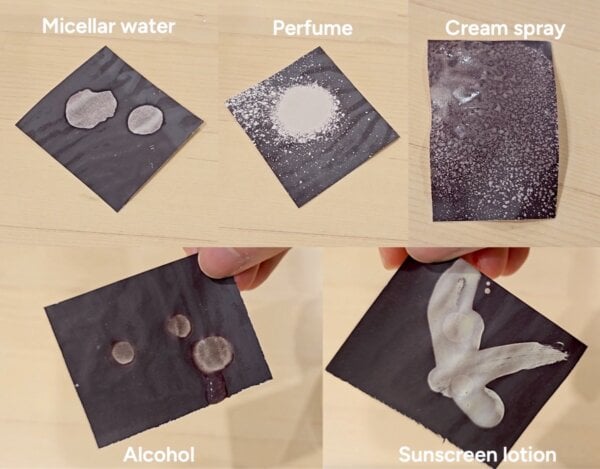

Some products didn’t work as well for this – I suspect it was to do with how fluid they were, and the fact that it’s harder to break up the intact ink than it is to keep the separate ink components apart.
This receipt test mostly shows which products are good at dissolving the top layer of receipts, and to a lesser extent, which receipts were still wet. It has nothing to do with which heat protectant works best on hair.
To work this out, you should test on actual hair. For example, one brand (Amika) whose product “failed” the receipt test showed footage of their actual testing, which involves combing samples of treated hair repeatedly in a sort of hamster wheel machine:


Heat protectants don’t work by blocking heat. In theory they should spread out heat so the hair heats up more evenly, to avoid hot spots – although there’s also an argument that they only work by conditioning hair, so it doesn’t get as damaged by snagging in hot tools (but that’s a topic for future Michelle).
For heat protectants, it’s best to look for products from brands that do proper tests on the final formula. Look for specific claims that indicate this on the packaging (specific temperatures, extra information about tests e.g. “instrumental test”, “vs non-conditioning shampoo alone”). Effective ingredients include silicones, polymers and hydrolysed proteins. If they contain water, make sure it has time to dry before using a hot tool.
Related post: How do heat protectant hair products work?