Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124

How to cite:
Wong M. Hair porosity tests are a lie. Lab Muffin Beauty Science. January 28, 2026. Accessed January 28, 2026.
https://labmuffin.com/hair-porosity-tests-are-a-lie/
There’s a lot of haircare advice based around the idea of “hair porosity”. This is a pretty complex term that can mean a lot of different things in different contexts (this might become a longer deep dive one day!). A lot of the advice does lead people to products that suit their hair better, but this is mostly just coincidence – a lot of the ideas around porosity don’t make a lot of sense.
This article is adapted from my video on hair hydration. Also check out Part 1 on hair and water more broadly, and Part 2 on the myth of hygral fatigue.
A lot of the myths around porosity stem from the misconception that hair is ideally waterproof: in undamaged hair, the cuticle is thought to seal out water, and conditioners mimic is function for damaged hair. This isn’t true!
Undamaged hair can absorb about 30% (almost one-third) of its own weight in water, in minutes. The water content of undamaged, conditioned hair also changes rapidly, depending on humidity.
| Relative humidity (%) | Weight of water absorbed (%) |
|---|---|
| 0 | 0 |
| 8 | 3.9 |
| 40 | 10.2 |
| 63 | 14.8 |
| 86 | 22.6 |
| 100 | 31.2 |
From Robbins CR. Chemical and Physical Behavior of Human Hair. Springer Berlin Heidelberg 2012.
This is because hair’s natural conditioning F-layer is only on the top of each cuticle scale – there are lots of gaps where water can get in (hair is a lot like a pinecone).
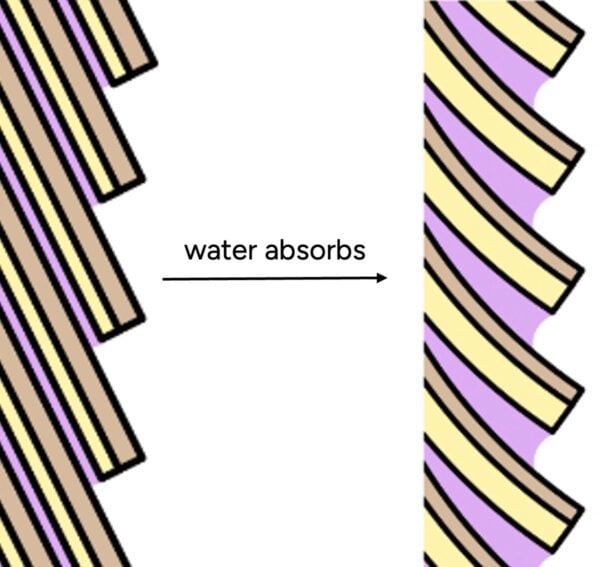
Conditioners also don’t seal out water. They deposit in blobs, not one continuous layer.
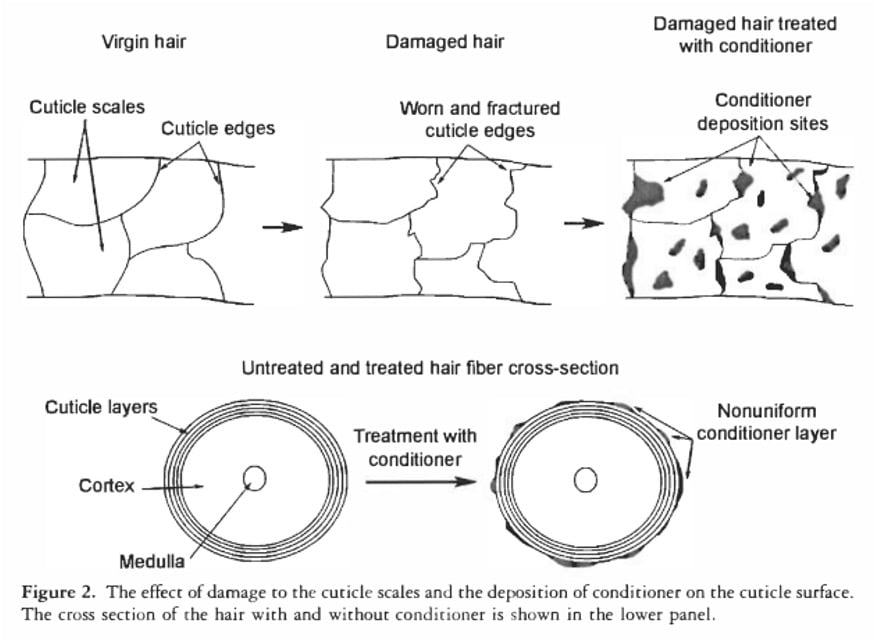

The conditioner blobs are small enough to make hair feel smooth and soft when we touch it. But individual water molecules are tiny, so the conditioner blobs aren’t very effective at keeping water out.
This might be confusing if you’ve seen tests that claim to diagnose your hair’s porosity. Examples:
The Float Test: Place a strand of hair in a glass of water. More damaged hair sinks, while less damaged hair floats. This is explained by the “high porosity hair” sinking when it’s absorbed enough water.


The Drop Test: Place a drop of water on a lock of hair. On undamaged hair it stays in a nice round bead, but it flattens out on damaged hair. This is explained by the water absorbing into “high porosity” hair because it’s full of holes.


If undamaged hair isn’t waterproof, what’s going on?
The answer is surface tension. These observations are actually to do with how water interacts with the surface of hair, not whether or not it soaks inside!
In liquid water, the individual water molecules really like holding hairs with each other through hydrogen bonds. The water molecules at the top have less neighbours to hold hands with, so it’s like they have extra hands grabbing along the surface.
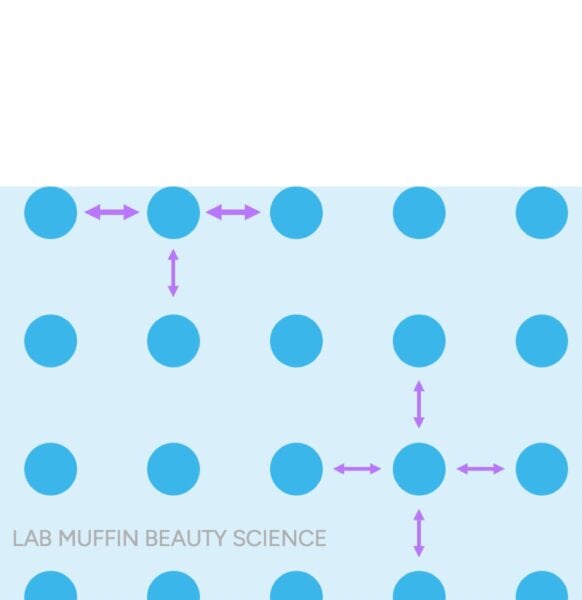

This forms a sort of strong “skin” that can hold up insects and hair and paperclips, even though they’re all denser than water (steel is about 8 times denser!):


If you poke at the paper clip or add a drop of detergent, the paperclip sinks, even though it’s made of solid steel and can’t absorb any water. Both hair and the paper clip are dense enough to sink in water already… but they only sink if the surface tension is disrupted.
In undamaged hair, there’s an oily layer covering the surface of each cuticle scale (the F-layer). But if the hair surface is damaged, this layer is removed, leaving a surface that likes water (hydrophilic).
When the damaged hair surface meets water, it holds hands with surface of the water through hydrogen bonds. This means there’s less extra hands making a strong “skin”, so the hair sinks.
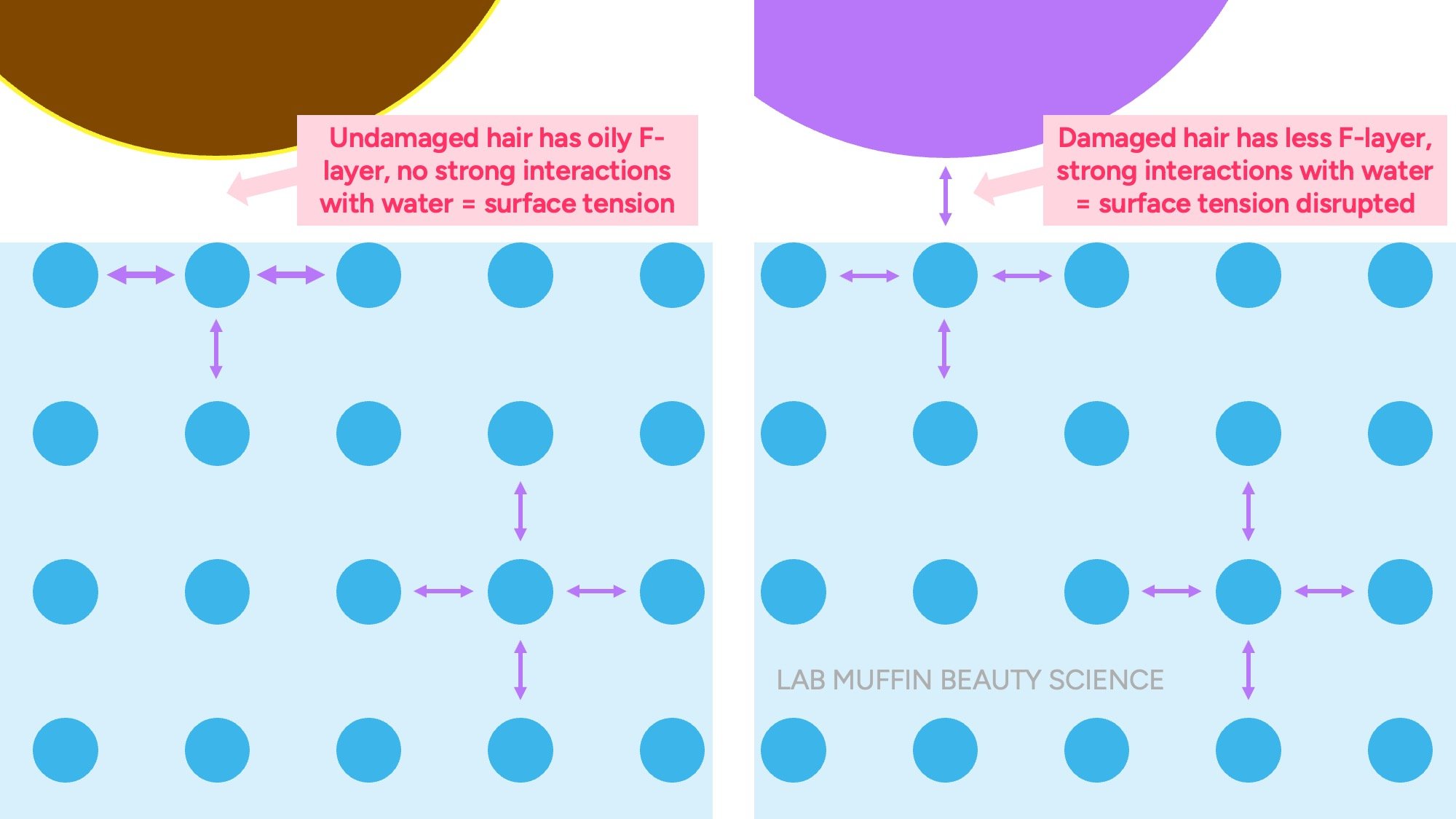

Surface tension also explains the drop test.
Damaged hair can absorb somewhere around 45% of its own weight in water, which isn’t much more than the 30% for undamaged hair. So the difference isn’t because water absorbs into the damaged hair – the water is actually spreading along the surface of the hairs and between them.
So how is this 30% weight of water getting into undamaged hair? It’s because this water isn’t liquid water – it’s gas.
Remember from high school science that in a gas, the particles aren’t holding hands. In humid air, individual water molecules are floating around – these are small enough to wiggle in between the cuticle scales inside the hair, without surface tension getting in the way.
These tests actually check for damage to the surface of hair – more surface damage gets diagnosed as “high porosity”, while less surface damage gets diagnosed as “low porosity”. Haircare based on surface damage is largely sensible!
But since these tests don’t measure porosity directly, it means some conclusions don’t make a lot of sense. For example, chemical treatments may take a lot time to soak into your hair, but these tests say your hair is “high porosity” because you have a lot of surface damage. Or someone may be using lots of oils on their hair which leads to a “low porosity” result.
Most of the time this doesn’t make a huge difference, but if you’re a hairdresser – please don’t use these tests to judge how long you need for chemical treatments! The best test is to directly use the product on a strand of the person’s hair.
Robbins CR. Chemical and Physical Behavior of Human Hair. 5th ed. Springer Berlin Heidelberg 2012.
La Torre C, Bhushan B. Nanotribological effects of silicone type, silicone deposition level, and surfactant type on human hair using atomic force microscopy. J Cosmet Sci. 2006;57(1):37-56.